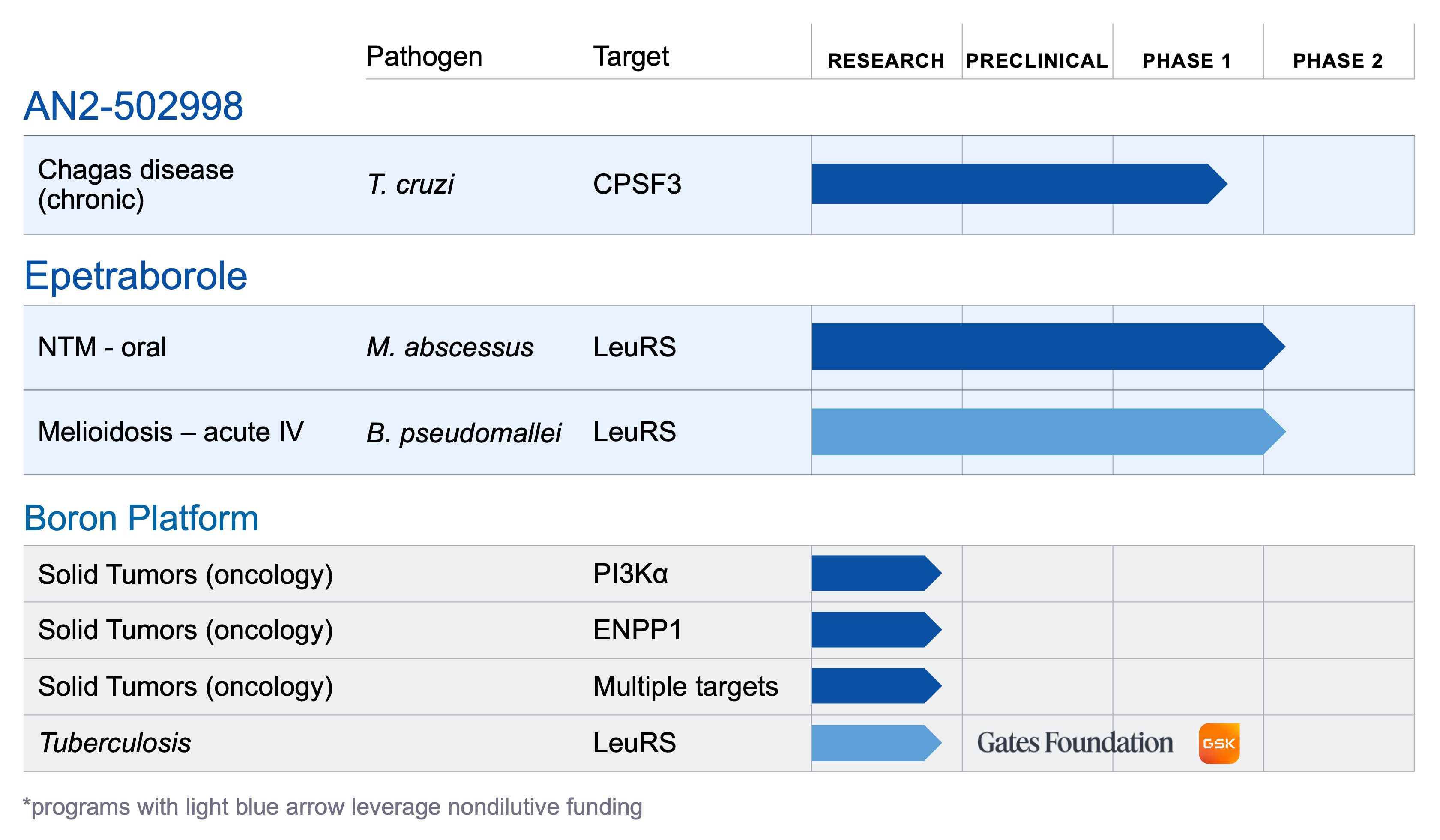
About AN2-502998
AN2-502998 (formerly known as AN15368) is a boron-based small molecule therapeutic candidate under development for the treatment of chronic Trypanosoma cruzi (T. cruzi) infection, which causes Chagas disease. AN2-502998 was originally discovered by researchers at Anacor, in close collaboration with the University of Georgia, and with grant funding from Wellcome. AN2-502998 is the only compound of which we are aware to have demonstrated curative activity in preclinical studies across multiple species, including in non-human primates with long-term, naturally acquired chronic infections of diverse T. cruzi genetic types. A Phase 1 clinical study is underway evaluating the safety, tolerability, and pharmacokinetics of oral AN2-502998 in healthy volunteers.
About Chagas Disease
Chagas disease is caused by the parasite Trypanosoma cruzi, which spreads via triatomine bugs (vector), a subspecies of blood-feeding insects more commonly known as “kissing bugs” because they tend to bite people on the face and lips. T. cruzi is also transmitted congenitally from infected mothers to their babies, through consumption of contaminated food or beverages, and via blood transfusions and organ transplants. While the disease can progress slowly, chronic infection almost inevitably results in irreparable damage to heart and digestive system tissues. If untreated, infection is lifelong and can be life threatening. Chagas disease kills more people in Latin America than any other infectious disease–including malaria, tuberculosis, and HIV—and is one of the major causes of infection-induced myocarditis or cardiomyopathy worldwide. An estimated 30% of Chagas patients develop chronic and often severe heart disease that leads to premature death.
According to the World Health Organization, approximately 6-7 million people worldwide are estimated to be infected with the parasite T. cruzi, with approximately 300,000 people infected in the U.S. and over 100,000 in Europe.
Chagas disease presents in an acute phase (~2 months after infection) and a chronic phase, where the T. cruzi parasites are hidden in muscle tissue, including the heart and digestive system. For over 50 years, two nitroheterocyclic compounds, benznidazole and nifurtimox, have been available for treatment of the infection and more recently were FDA approved for use in children, but are rarely used due to their inconsistent efficacy and high frequency of side effects. There are currently no approved therapies to cure the disease once it reaches the chronic phase; however, benznidazole and nifurtimox may be offered to people younger than age 50 because they may help slow the progression of the disease and its most serious complications.